Quality and safety of food materials are key factors in preserving and improving people’s health. All of us consume food daily, and we all should be confident that those foods are safe for us and our families.
Glycidyl esters (GEs) are produced from diacylglycerides in extreme heat (their content correlates with diacylglycerides level). Mono- and diacylglycerides are generated in oils by hydrolysis, caused by active lipases. Oil crop seeds and fruits contain lipase, an enzyme that gets more active with ripening. Lipase interacts with oil contained in ripe fruits, degrading triglycerides swiftly to free fatty acids, monoacylglycerides and diacylglycerides. Model systems show clear connection between those agents’ content and the subsequent glycidyl esters generation (Fig. 1) during heat treatment. For vegetable oils, factors promoting 3 MCPD and GEs generation during processing are: climate, soil, plant growth conditions, harvesting and storage methods
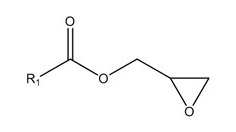
Alongside glycidyl esters of fatty acids, processing also produces 3 MCPD and 2 MCPD: compound esters of fatty acids (Fig. 2,3).
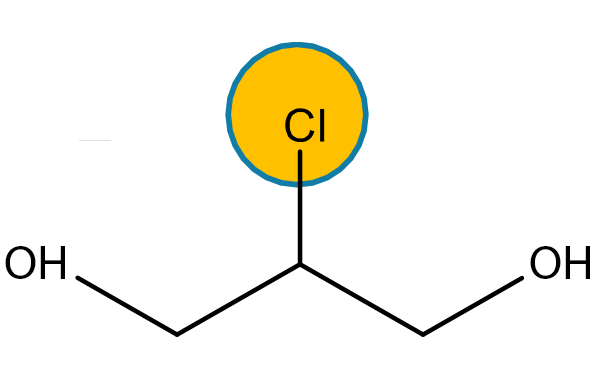
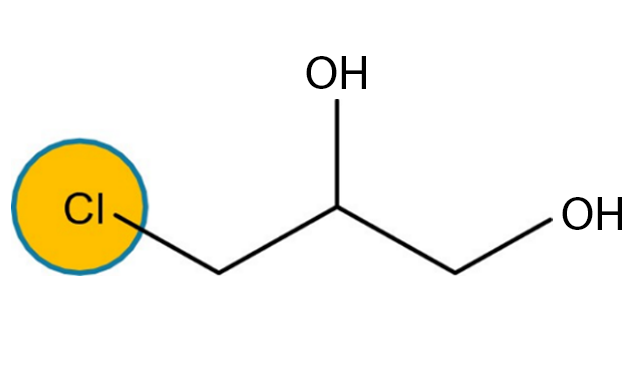
3 MCPD and 2 MCPD are organic compounds of the chloropropanediol family generated under heat through interaction of choline ions with lipids contained in oil as natural (i.e. chloroalkane) or technical (i.e. traces of hydrochloric acid) chlorine-containing compounds. The mechanism thanks to which 3 MCPD, 2 MCPD and GEs are formed during the oil refining process is not entirely clear yet.
A study conducted by Chemisches und Veterinäruntersuchungsamt (CVUA, Stuttgart, Germany) demonstrated that traces of 3 MCPD esters could be found in some natural unrefined fats and oils. Meanwhile, significant levels of 3 MCPD esters have been detected in almost all refined fats and oils.
As a rule, vegetable oils have to be refined before use, depending on the specific oil and the level of extraction contamination, as well as on, i.e., the desired sensory parameters of the refined oil. Glycidol esters are generated in vegetable oils and fats that are processed (refined) at temperatures above 200 °С. Free 3 MCPD and GE content is very low. Deodorization is considered the key refining stage that leads to 3 MCPD and glycidol esters production. However, it was also discovered that some contaminated matter is generated by bleaching, for example, with bleaching earth. Another discovery was that raw oil pre-treated with acids (hydrochloric or phosphorus) as a part of the refining process, boosts up 3 MCPD esters generation.
3 MCPD and GE compound esters are present in many foods. They are generated by smoking, roasting, grill or frying. 3 MCPD and GEs get into foods, including confectionery and milk-containing products, such as baby food, sauces, spreads, cream and milk replacers, ice cream, with some
ingredients: vegetable oils and fats. Sources classify vegetable oils into three groups, depending on their glycidyl esters content:
The higher the diacylglycerides level in the oil, the higher its GEs content.
Free chloropropanediols, 3 MCPD in particular, were detected in many soy-based products, including, for example, soy sauce and acid-hydrolyzed plant protein. When consumed with food, compound esters of fatty acids: chloropropanediols and glycidol, are hydrolyzed by lipases in the gastrointestinal tract, releasing free chloropropanediols and glycidol which are, as studies prove, genotoxic and carcinogenic.
The majority of research accumulated by now suggest that presence of glycidyl esters of fatty acids and glycidol in food is a risk for human health, especially for children’s health. According to the International Agency for Cancer Research (IACR), glycidol is a carcinogen.
As IACR estimates:
European Commission issued regulation limiting fatty acid GE content in vegetable oils and fats intended for retail or as food material, to 1 mg/kg of product (Table 1).
Table 1. Maximum GE levels for some foodstuffs
| Регламент комиссии европейских сообществ № 1881/2006 с учетом изменения №2018/290 от 26 февраля 2018 года | Максимально допустимый уровень ГЭ в пересчете на глицидол |
|---|---|
| Растительные масла и жиры, предназначенные для непосредственного потребления человеком или использования в качестве ингредиента в продуктах питания | 1 мг/кг |
| Растительные масла и жиры, предназначенные для производства детского питания | 0,5 мг/кг |
| Смеси для детского питания, смеси для последующего использования и продукты медицинского назначения, предназначенных для младенцев и детей младшего возраста (порошок) | 0,075 мг/кг |
| Смеси для детского питания, смеси для последующего использования и продукты медицинского назначения, предназначенных для младенцев и детей младшего возраста (жидкие) | 0,01 мг/кг |
According to SanPiN 1.2.2353-08 Carcinogenic factors and main requirements for cancer hazard prevention, glycidol is a carcinogenic chemical. Based on that, the EAEC Collegium adopted the following maximum content levels for this substance (Table 2):
Table 2. Maximum GE levels for some foodstuffs
| Единые санитарно-эпидемиологические и гигиенические требования к продукции (товарам) подлежащей санитарно-эпидемиологическому контролю, внесены решением Коллегии Евразийской экономической комиссией от 06.08.2019 №132 | Максимально допустимый уровень ГЭ в пересчете на глицидол |
|---|---|
| Масла растительные, предназначенные для непосредственного употребления человеком в пищу и в качестве продовольственного (пищевого сырья) | 1 мг/кг |
| Масло подсолнечное, соевое, кукурузное, используемые при изготовлении продуктов детского питания | 0,5 мг/кг |
| Сухие адаптированные молочные смеси и продукты на основе частично гидролизованных белков, содержащие немолочные жиры | 0,05 мг/кг |
| Жидкие адаптированные молочные смеси и продукты на основе частично гидролизованных белков, содержащие немолочные жиры. Специальные продукты для лечебного питания детей, содержащие немолочные жиры. | 0,006 мг/кг |
On September 23, 2020, the European Commission adopted Regulation 2020/1322, amending Regulation (EC) 1881/2006 regarding maximum levels of monochloropropanediol and 3 MCPD compound esters: 2.5 mg/kg for vegetable oils and 1.25 mg/kg for baby food. Now, the glycidol, 3 MCPD and their compound esters content must be controlled in foods listed in the Regulation, if they are sold in or exported to the EU.
ДQuantitative assessment of glycidol, 3 MCPD and their compound esters content is possible in two ways: direct assessment of glycidyl and 3 MCPD fatty acid esters by HPLC-MS and indirect assessment by GC-MS. The direct method has one significant drawback: lots of compound esters combinations make it necessary to handle lots of chromatography peaks, and their recognition is problematic due to lack of analytical standards. The indirect method involving breaking up and derivatization of compound esters, yields three compound peaks that can be easily identified and assessed using available analytical standards.
One of the first assessment methods adopted by International Organization for Standardization is SO18363-1: 2015 (AOCS Official Method Cd 29c-13, GOST ISO 18363-1-2020) method providing for quantitative assessment of 3 MCPD content through alkalic breakdown of 3 MCPD esters and glycidol to 3 MCPD. Glycidol esters (glycidol equivalent) were assessed by recalculating the difference in quantitative results of two injections. The main drawback of that method is unprecise quantitative assessment of glycidol, because it presumes that there are no other substances interacting with inorganic chloride to produce 3 MCPD. The relative standard deviation for one sample test in different laboratories if 3 MCPD content is close to 1 mg/kg is over 25%; for 3 MCPD content close to 0.1 mg/kg – over 130%. Moreover, there is no way to assess 2 MCPD content using this method. But the method is easy to automate and has very fast breakdown time, which is great for production labs, which are mainly focused on production processes adjustments.
For that reason Saratov combined works laboratories adopted the GOST ISO 18363-1-2020 method for MCPD 3 and GE compound esters assessment. We use gas chromatography device Аgilent 7890В with mass-selective detector Аgilent 5977В, as well as MPSrobotic sample preparation and dosing robot by GERSTEL (Germany) for contaminant analysis. The fully automated sample preparation process is based on DGFC-Ⅵ 18 method similar to ISO18363-1: 2015; AOCS Official Method Cd 29c-13; GOST ISO 18363-1-2020 methods. Automated test samples preparation saves time and reduces human error by eliminating manual operations. The robot concentrates the samples to bring down detection limit and changes the diluent to the one more suited to the GC-MS chromatography and ionization conditions.
The next methods to get standardized were ISO 18363-2: 2018 (AOCS Cd 29b-13, GOST ISO 18363-2-2020) and ISO 18363-3: 2017 (AOCS Cd 29а-13, GOST ISO 18363-3-2020). Both of them are much more precise than the first one, but their breakdown reaction time
is significant: over 16 hours. That limits their viability for production severely, but due to direct quantitative assessment, they provide more precise data to the laboratories issuing test certificates and are liable for their results. Besides, the ISO 18363-3:2017 method is hard to automate, but reproduced better in other laboratories, while ISO 18363-2: 2018 requires two sample preparations and two injections.
Tougher requirements to food quality and safety and developing food market globalization make oil&fats producers tackle new challenges. One of those challenges is to minimize the content of monochloropropanediol and glycidol compound esters potentially harmful for human health in vegetable oils. In the EU, Commission Recommendation 2014/661/EU dd. September 10, 2014 is in effect, requiring to monitor the contents for the compounds in question in foodstuffs.
The contaminant minimization issue can only be solved if we understand the processes that form monochloropropanediol and glycidol compound esters in oils, master the test methods and, based on the knowledge we get, develop the best action strategy.
Probably, monochloropropanediol compound esters are generated in food-grade vegetable oils as follows: chlorine is an element relatively common in nature. That’s why it could be safe to assume that a broad variety of chlorine sources, both organic and inorganic, could be potential precursors for monochloropropanediol compound esters generated when food-grade vegetable oils are produced. Lipids included in the oils, i.e. acyl glycerols, phospholipids and glycolipids, could theoretically react with chlorine-containing compounds during oil refining, producing 3 MCPD compound esters. The precursor nature would no doubt depend on the type of oil, its quality and production technology. Authors of Zelinkova, Z. Fatty acid esters of 3-chloropropane-1,2-diol in edible oils posit that the organic chlorine compounds detected are similar in their structure to phytosphingosine and are endogenous plant metabolites, not exogenous contaminants. Therefore, the main populations of chlorine donors may vary depending on material batch, and that will subsequently play a role in the course of 3 MCPD compound esters generation process.
When it comes to the most probable lipid precursors of 3 MCPD in vegetable oils, the first to be suggested was partial acyl glycerols, in particular, diacylglycerols (DAGs). The basis for the suggestion was the fact that the highest 3 MCPD content was detected in oils produced from flesh of oil fruits, such as palm or olive oil. It is a common fact that those oils have elevated DAGs content produced by hydrolysis of lipolytic enzymes. But further studies disproved that hypothesis. They showed that although DAGs may theoretically interact with chlorine donors and produce 3 MCPD compound esters, they are nevertheless not the main lipid precursors of that process because it has been proven that the bulk of 3 MCPD compound esters is generated at the deodorization stage. Other excipient lipids, such as phospholipids or glycolipids, also can’t participate in production of 3 MCPD compound esters to any significant extent, because they are eliminated at the preceding stages of refining.
In order to ascertain the origins of chlorine producing 3 MCPD compound esters during refining, the work Hrnčiřík, K Aninitial study on the formation of 3 MCPD esters during oil refining studied the content of chlorine ions in oil before and after deodorization, in order to detect a correlation between that value and the 3 MCPD compound esters content in refined oil. But no such correlation was uncovered. An attempt to explain production of 3 MCPD compound esters during deodorization through chlorine presence in direct steam also failed. And only Nagy, K. Mass-detect filtering of isotope signature store veal the source of chlorinated palm oil contaminants revealed the correlation between heat-induced breakdown of organic chlorine compounds in palm oil and 3 MCPD accumulation during heat treatment of palm oil. The authors tracked the breakdown of organic chlorine compounds detected by high performance liquid chromatography and mass-spectrometry in unrefined palm oil and discovered that the typical product of those compounds’ breakdown was hydrochloric acid (HCl), which is the main chlorine donor for 3 MCPD compound esters production during deodorization.
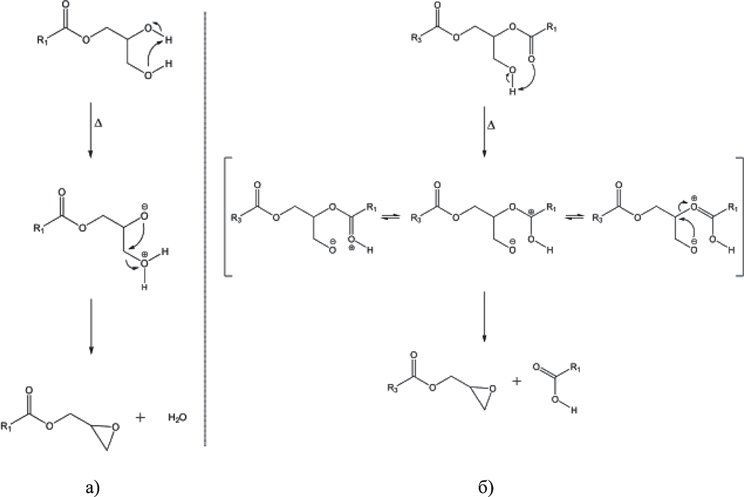
Therefore, according to the conclusions drawn by F. Destaillats, there are now two theoretically acceptable ways to generate 3 MCPD compound esters (Fig. 4).
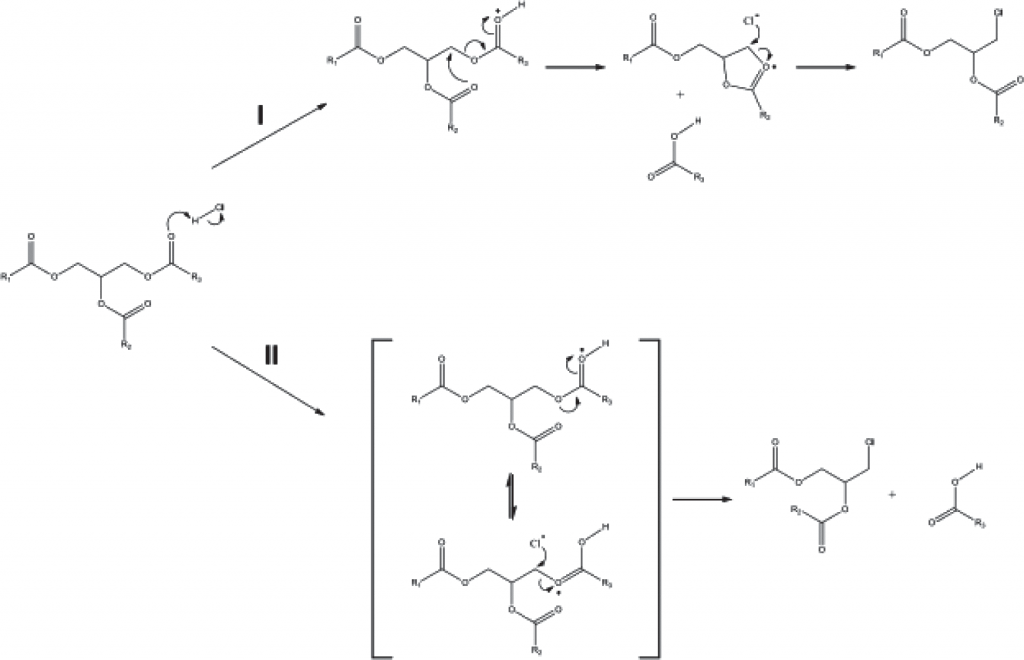
However, the author disproved the assertion concerning the precursors for 3 MCPD and GE compound esters production being the same. In particular, he proved that precursors for glycidol compound esters formation in oil in extreme heat (200 °С or above) are mono- and diacylglycerols. It’s worth noting that insignificant increase in glycidol compound esters content can be observed even at the temperatures above 180 °С, but above 200 °С, there is a sharp increase in glycidol compound esters accumulation rate in oil. See Fig. 5a and 5b, respectively for the processes of glycidol compound esters formation from mono- and diacylglycerols in extreme heat (200 °С or above) developed by the authors.
Based on the above, we can reduce the content of 3 MCPD and glycidol compound esters in vegetable oils by utilizing the following approaches and methods:
Many studies suggest a direct correlation between bleaching clay acidity level and 3 MCPD compound esters content in refined oil. Based on that, it was proposed to use natural and acid-activated bleaching clays with more neutral pH values in oil refining, in order to reduce 3 MCPD compound esters production. Flushing is also recommended to remove the traces of mineral acids.
Many studies suggest a direct correlation between bleaching clay acidity level and 3 MCPD compound esters content in refined oil. Based on that, it was proposed to use natural and acid-activated bleaching clays with more neutral pH values in oil refining, in order to reduce 3 MCPD compound esters production. Flushing is also recommended to remove the traces of mineral acids.
Considering that in addition to temperature, the duration of deodorization process also plays a role, multiple studies agreed on a suggestion to use the so-called two-stage deodorization. A similar approach has already been used on industrial scale to reduce fatty acids trans isomers production and optimize tocopherols level in oils. Combining a short high temperature deodorization stage at high temperature (250/270 °С) with a longer stage at a lower temperature (200 °С) can help reduce heat load on the oil. For example, combining deodorization at 200 °С for 120 minutes with subsequent deodorization at 250 °С for 5 minutes reduces 3 MCPD compound esters and related compounds content by one third, and 3 MCPD compound esters alone by two thirds compared to traditional deodorization. A two-stage deodorization at 270 °С combined with a preceding long deodorization at a lower temperature reduces 3 MCPD compound esters and related compounds content by almost 80% (Matthäus, B. Final results of the German FEIResearch Project Concerning 3-MCPD Esters and Related Compounds). Another effective way to reduce heat load on oil, and, most importantly, to virtually eliminate production of 3 MCPD and glycidol compound esters is using molecular distillation instead of deodorization or together with it in mild conditions. The biocathalytic method allowing for elimination of 3 MCPD esters from refined oils
through fermentation processes is also promising (Bornscheuer, U. T. Enzymatic removal of 3-monochloro-1,2-propandiol (3-MCPD) and its esters from oils).
When using these strategies in practice, it is important to find the balance between maximum possible reduction of 3 MCPD and GE compound ester contaminants during processing and preserving the oil quality.